Nuvaxovid
Novavax is approved and available for use as a booster in. Full results from Nuvaxovids pivotal phase III trial were published in December 2021.

Middlesex London Health Unit Are You Interested In The Novavax Nuvaxovid Covid 19 Vaccine Mlhu Is Receiving A Limited Supply Of The Vaccine This Spring Call 1 226 289 3560 To Be Added To The Waitlist
Vaccination efforts a needed boost.

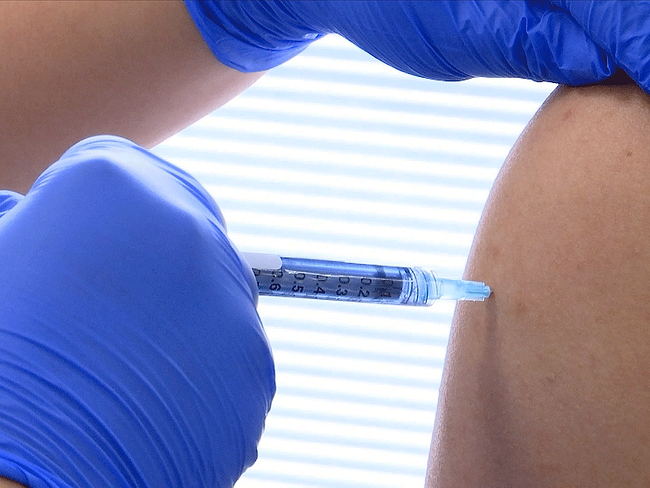
. Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. 10 hours agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Your doctor pharmacist or nurse will inject the vaccine into a muscle intramuscular injection in your upper arm.
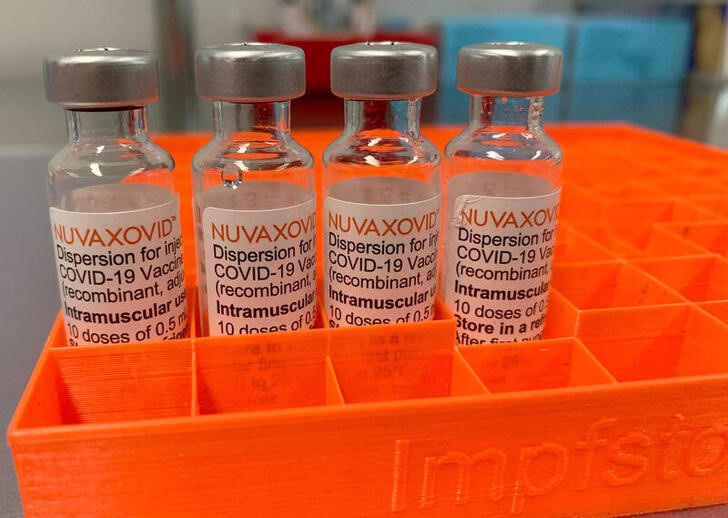
Nuvaxovid is the first protein-based COVID-19 vaccine granted authorization from the Medicines and Healthcare products Regulatory Agency MHRA and will be offered per advice from the Joint Committee on Vaccination and Immunisation. This is a multidose vial. Nuvaxovid Novavax is approved and available for use as a primary course in people aged 12 years and over.
Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022. As such HSA will be monitoring the incidence rate of pericarditis or inflammation of the outer lining of the heart and myocarditis. How is NUVAXOVID given.
Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Det eftersom att data. The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency.
The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. Company Novavax should not be given to individuals. Nu stoppar Folkhälsomyndigheten användningen bland personer.
Qualitative and quantitative composition. COVID-19 Vaccine recombinant adjuvanted 2. Enligt testerna skulle det ju vara säkrare än Pfizer hur kan det komma sig att Pfizer används.
The Novavax COVID-19 vaccine sold under the brand names Nuvaxovid and Covovax among others is a subunit COVID-19 vaccine developed by Novavax and the Coalition for Epidemic Preparedness Innovations CEPI. Find detailed technical information such as the product monograph and. Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022.
After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further SARS-CoV-2 vaccine was approved by the European Medical Agency EMA in December 2021. This vaccine called Nuvaxovid produced by the company Novavax is a so. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes.
The Summary of Product Characteristics is a description of a. Nuvaxovid dispersion for injection. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19.
8 hours ago3rd November 2022 0355 GMT11. Name of the medicinal product. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant. During and after each injection of the vaccine your doctor pharmacist or nurse will watch over you for around 15 minutes to monitor for signs of an allergic reaction. HSAs assessment is that although the potential risk of myocarditis with Nuvaxovid COVID-19 vaccine cannot be excluded the benefits of the vaccine continue to outweigh the risks in the Singapore context it said.
The trial includes a demographically diverse population in the United States and Mexico and provides strong evidence of high short-term vaccine efficacy of NVX-CoV2373 for. In using an old standby technology Nuvaxovid vaccines dont have to be kept as cold as the. The Novavax Nuvaxovid COVID-19 vaccine was authorized for use in Canada under the Food and Drug Regulations.
11 hours agoPublicerad idag 0702. The addition of the saponin-based Matrix-M adjuvant facilitates activation of the cells of the innate immune system which enhances the magnitude of the S protein-specific immune response. Beslutet är temporärt och gäller från.
2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US. Nuvaxovid boosters could give the US. Vaccinationer med Nuvaxovid pausas för personer 30 år och yngre Folkhälsomyndigheten.
On December 20 2021 the. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten.

Novavax Says Initial 1m Doses Of Nuvaxovid Covid 19 Vaccine Are Available In Uk

Fda Advisers Overwhelmingly Endorse Novavax Covid 19 Vaccine Ars Technica

Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Gov Uk

Novavax Nvx Cov2373 Nuvaxovid In Europe And Australia Covovax In India And The Philippines Tak 019 In Japan

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl

Covid Vaccine Novavax Requests Who To Expand Emergency Use Listing Of Nuvaxovid For Adolescents Aged 12
U S Fda Authorizes Novavax Covid Vaccine For Adults Reuters

Ministry Of Health Singapore On Instagram Registration For The Nuvaxovid Vaccine By Novavax Has Begun Individuals Aged 18 Years And Above May Receive The Vaccine For Their Primary

Novavax Covid 19 Vaccine Nuvaxovid Data On Side Effects
Covid Vaccine Maker Novavax Drops After Cutting Sales Outlook 50 Nvax Bloomberg

Investigational Vaccine Candidate Novavax Covid 19 Vaccine

Ec Expands Novavax Nuvaxovid Covid 19 Vaccine Approval

Ema Plans Anaphylaxis Label On Novavax Covid 19 Vaccine
Novavax Makes One Million Doses Of Nuvaxovid Available For Use In The United Kingdom Pharmtech Focus

The Fda S Decision On The Novavax Covid 19 Vaccine Could Come In Weeks Marketwatch
Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Pharmatutor
New Protein Based Covid 19 Vaccine Could Help Boost Rates Say Pharmacists Cbc News